Three New Cancer Treatments Approved by the FDA
Last week saw a flurry of new anticancer therapeutics approved by the U.S. Food and Drug Administration (FDA) for treating several types of cancer. On Monday, Sept. 24, the agency approved the molecularly targeted therapeutic duvelisib (Copiktra) for treating certain patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), and for treating certain patients with follicular lymphoma. On Thursday, Sept. 27, it approved another molecularly targeted therapeutic—dacomitinib (Vizimpro)—for treating certain patients with non–small cell lung cancer (NSCLC). Then, on Friday, Sept. 28, it approved the immunotherapeutic cemiplimab-rwlc (Libtayo) for treating certain patients with cutaneous squamous cell carcinoma.
A new molecularly targeted therapeutic for hematologic cancers
Hematologic cancers arise in blood-forming tissue, such as the bone marrow, or in cells of the immune system.
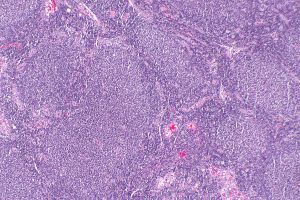
Follicular lymphoma is a slow-growing type of non-Hodgkin lymphoma that arises in immune cells called B cells. Image by Nephron via Wikipedia.
Non-Hodgkin lymphoma is the most common hematologic cancer diagnosed in the United States. In 2018, 74,680 people in the United States are expected to be newly diagnosed with the disease, according to data from the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results Program (SEER). About 20 percent of these cases will be classed as follicular lymphoma, which is a slow-growing type of non-Hodgkin lymphoma that arises in immune cells called B cells.
CLL and SLL are also slow-growing hematologic cancers that arise in B cells. According to the NCI, CLL and SLL are essentially the same disease, but have different names depending on where in the body the cancer cells accumulate. CLL cells are found mostly in the blood and bone marrow, whereas SLL cells are found mostly in the lymph nodes. The latest SEER data show that the number of new cases of CLL/SLL was 4.3 per 100,000 U.S. men and women per year.
Duvelisib targets two forms of a molecule called phosphatidylinositol 3-kinase (PI3K), PI3K-delta and PI3K-gamma. PI3K is a component of a signaling pathway that has a key role in promoting the survival and expansion of many types of cells, including the B cells affected in CLL/SLL and follicular lymphoma. PI3K is, therefore, a prime therapeutic target for these hematologic cancers.
Duvelisib was approved by the FDA only for patients with CLL/SLL and follicular lymphoma whose disease has progressed after they have received at least two other types of treatment.
The approval for CLL/SLL was based on results from a randomized phase III clinical trial, which showed that a significantly greater proportion of patients responded to treatment with duvelisib compared with the standard treatment, ofatumumab (Arzerra). The overall response rate was 78 percent among the 95 patients randomized to duvelisib compared with 39 percent among the 101 patients randomized to ofatumumab. The median progression-free survival was also longer among those randomized to duvelisib. It was 16.4 months in the duvelisib arm compared with 9.1 months in the ofatumumab arm.
The approval of duvelisib for follicular lymphoma was based on results from one arm of a randomized phase III clinical trial. The results showed that 35 of 83 patients whose disease was not responding to standard treatments (42 percent) responded to treatment with duvelisib. One of these patients had a complete response, the rest had partial responses.
A new molecularly targeted therapeutic option for NSCLC
Mouse models of lung cancer, such as the one shown here, are important research tools for driving progress against the disease. Image courtesy of National Cancer Institute.
More than 1.75 million people around the world are expected die from lung cancer in 2018, making it the most commonly diagnosed cancer globally. About 154,050 of these deaths will occur in the United States, according to data from the NCI SEER program. These statistics highlight the need for new approaches to treating lung cancer.
NSCLC is the most common type of lung cancer diagnosed in the United States. Research has shown that mutations in the EGFR gene are one of the most common drivers of the disease. With the approval of dacomitinib, there are now five EGFR-targeted therapeutics approved for treating certain patients with NSCLC testing positive for EGFR mutations; the other four are afatinib (Gilotrif), erlotinib (Tarceva), gefitinib (Iressa), and osimertinib (Tagrisso).
The FDA approved dacomitinib as an initial treatment only for patients with metastatic NSCLC that tests positive for certain EGFR mutations, either an EGFR exon 19 deletion or the exon 21 L858R mutation.
The approval was based on results from the randomized, phase III ARCHER 1050 clinical trial, which were published last fall in Lancet Oncology. In brief, dacomitinib significantly improved progression-free survival for patients compared with gefitinib. The median progression-free survival was 14.7 months in the dacomitinib arm compared with 9.2 months in the gefitinib arm. The approval was granted even though the researchers did not find any improvement in overall survival.
A first checkpoint inhibitor for cutaneous squamous cell carcinoma
Squamous cell carcinoma is the second most common type of skin cancer. There are about 700,000 cases of the disease diagnosed each year in the United States, according to the FDA. Fortunately, most patients are cured by surgery and/or radiation. The emergence of advanced disease is rare. However, if it does occur, it can be difficult to treat and there were no therapeutics approved specifically to treat it before last Friday’s approval of cemiplimab-rwlc.
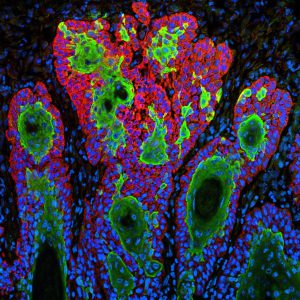
Squamous cell carcinoma is the second most common type of skin cancer. Image courtesy of the National Cancer Institute.
The approval of cemiplimab-rwlc for treating patients with metastatic cutaneous squamous cell carcinoma or locally advanced CSCC that cannot be treated with curative surgery or curative radiation was based on results from two small clinical trials. Overall, 47.2 percent of the 108 patients who received cemiplimab-rwlc responded to the immunotherapeutic.
Cemiplimab-rwlc is a type of immunotherapy known as a checkpoint inhibitor. It is the seventh checkpoint inhibitor approved by the FDA, and the sixth to target the PD-1/PD-L1 pathway that puts the brakes on cancer-fighting immune cells called T cells. Two researchers whose work was critical to the development of these immunotherapeutics, which have revolutionized the treatment of 13 types of cancer and the treatment of solid tumors positive for either the MSI-hi or MMR-deficient biomarkers, were recognized this week for their pioneering work in cancer immunotherapy with the 2018 Nobel Prize in Physiology or Medicine.
Read more about the Nobel Prize winning researcher, Fellow of the AACR Academy, James P. Allison, PhD, in another post on this blog.




Excellent summaries