Targets Conference Showcases Exciting Data from Clinical Trials of Innovative Cancer Therapeutics
There has been an explosion in the number of cancer therapeutics and clinical trials in the recent past, owing to our ability to better define the molecular targets of different cancers using cutting-edge technologies. Unlike in the past, data from early-stage clinical trials are getting more attention lately because the efficacy of a therapeutic, traditionally evaluated in later-phase trials, is often becoming evident earlier in the course of clinical testing.
Rather than “one size fits all” treatment approaches, the new wave of therapeutics is increasingly precise, and many patients’ tumors are matched to a genomically targeted treatment. Because of this, the clinical activity of such treatments is often measurable even at suboptimal doses in a phase I trial. Such early data offer a glimpse into the future of the therapeutic, exciting all stakeholders in the oncology field.
The AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, cochaired by William Sellers, MD, of the Broad Institute of MIT and Harvard, Harvard Medical School, and the Dana-Farber Cancer Institute; James L. Gulley, MD, PhD, chief of Genitourinary Malignancies Branch and director of Medical Oncology Service at the National Cancer Institute; and Emiliano Calvo, MD, PhD, director of clinical research at START Madrid, Hospital de Madrid Norte Sanchinarro, is “the home of early-phase oncology drug development,” as noted by Calvo in a media briefing.
A number of early-stage clinical trials discussed at the meeting tested innovative therapeutics against rare and hard-to-treat cancers, including advanced head and neck squamous cell cancer (HNSCC), pancreatic ductal adenocarcinoma (PDAC), and plexiform neurofibromas.
Data from a clinical trial and preclinical studies of innovative KRAS inhibitors presented at the conference are discussed in this blog post.
Promising results from a HRAS-targeted therapeutic for patients with advanced head and neck cancer
This year, about 53,000 people will be diagnosed with cancers of the oral cavity and pharynx (throat), and about 10,860 are expected to die from this disease, according to federal estimates. “Recurrent and/or metastatic head and neck squamous cell carcinoma (HNSCC) is an incurable and devastating disease for which new treatments are needed,” says Alan L. Ho, MD, PhD, medical oncologist at Memorial Sloan Kettering Cancer Center.
Ho presented data from a phase II clinical trial that tested the investigational farnesyltransferase inhibitor tipifarnib in patients with HNSCC. About 5 to 8 percent of patients with advanced HNSCC have tumors with an HRAS mutation, according to Ho.
Tipifarnib is a highly selective inhibitor of the enzyme farnesyltransferase, which activates the proto-oncogene HRAS. Although this therapeutic was initially developed more than a decade ago to target mutations in all three RAS genes, KRAS, HRAS, and NRAS, researchers learned that this mode of inhibition will not be effective against mutant KRAS and NRAS as these proteins could be activated though another mechanism that involves an enzyme called geranylgeranyltransferase. Further studies revealed why HRAS-mutant cancers may be uniquely susceptible to tipifarnib, Ho noted. Tipifarnib is the first and only compound to demonstrate activity against HRAS driven tumors, he added.
Data from the tipifarnib trial showed that patients with recurrent and/or metastatic HNSCC harboring HRAS gene mutations had durable objective responses to tipifarnib. Among the 15 patients with HNSCC that had HRAS missense mutations at a high variant allele frequency (VAF; 35 percent or higher), the overall response rate was 53 percent, which included eight partial responses and five stable disease.
The median progression-free survival (PFS) in HNSCC patients with a VAF of 20 percent or higher was 5.4 months; for those with a partial response, the PFS was 19 months; the PFS was 4.5 months for those with stable disease. This is striking, considering these patients had no objective responses on previous prior therapy, where the median PFS was 3.2 months.
A potentially tissue-agnostic therapeutic is effective against advanced pancreatic and lung cancer
Neuregulin 1 (NRG1) is a gene that encodes a membrane glycoprotein that mediates signaling between cells; the glycoprotein plays a key role in the growth and development of multiple organ systems. Analysis of large datasets from The Cancer Genome Atlas and MSK-IMPACT have shown NRG1 fusions occur in many cancer types, including breast, head and neck, renal, lung, ovarian, pancreatic, prostate, and uterine cancers.
While thought to occur in less than 1 percent of solid tumors, the true incidence may be higher, noted Alison Schram, MD, a medical oncologist in the Early Drug Development Service at Memorial Sloan Kettering Cancer Center (MSK). Certain subtypes of lung and pancreatic cancers may have higher rates of NRG1 fusions.
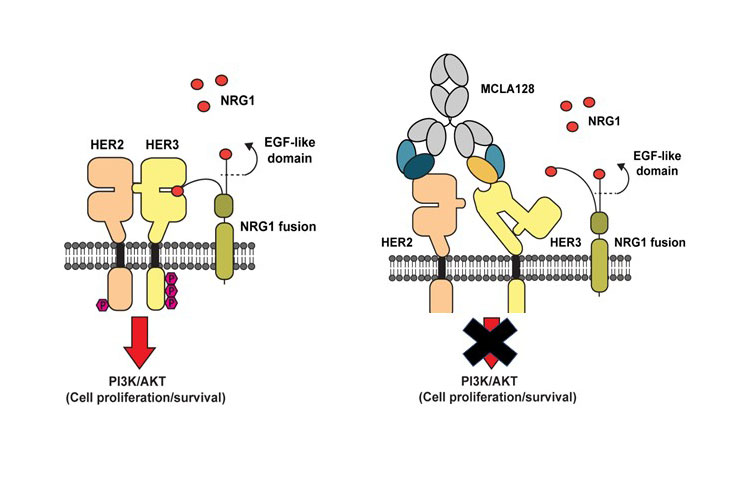
Schram presented data on patients treated with an investigational bispecific HER2/HER3 antibody therapeutic MCLA-128 through the U.S. Food and Drug Administration-approved single-patient investigational drug applications protocol initiated by MSK as part of an expanded access program.
NRG1 fusion proteins drive tumor growth by binding to HER3 receptors on the cell surface. HER3 forms a heterodimer with other HER-family receptors, including HER2, which activates a signaling pathway that causes cancer cell growth and proliferation. MCLA-128 has two arms, one that binds to HER2, physically impairing HER2 from binding to HER3, and another that binds to HER3 at the NRG1 binding site preventing NRG1 fusions from activating HER3, noted Schram.
Given that tyrosine kinase inhibitors (TKI) work by inhibiting HER2 from activating proteins downstream in the pathway but do not alter NRG1 binding to HER3, “MCLA-128’s mechanism of action is really a perfect fit for the genetic abnormality being targeted,” Schram said in an interview.
Data presented by Schram on three patients showed significant tumor shrinkage in two patients with ATP1B1-NRG1 fusion-positive metastatic PDAC and one patient with CD74-NRG1 fusion-positive metastatic non-small cell lung cancer (NSCLC). In the two patients with PDAC, treatment with MCLA-128 resulted in CA 19-9, a tumor biomarker for pancreatic cancer, dropping from 262 units/ml to 50 units/ml and 418 units/ml to 11 units/ml, respectively (the normal rage for this protein is less than 40 units/ml). The treatment was also effective in shrinking the patient’s liver metastases.
The patient with NSCLC had brain metastases and his disease had progressed on six prior lines of therapy, including the TKI afatinib (Gilotrif). Treatment with MCLA-128 led to tumor shrinkage by 33 percent with improvement in his brain metastases. “This emphasizes the potential superiority of this approach over TKIs,” Schram said. A phase II trial of MCLA-128 tested in patients with NRG1 fusion-positive tumors is underway.
A MAT2A inhibitor shows activity in patients with solid tumors lacking the MTAP gene
Rebecca S. Heist, MD, medical oncologist at Massachusetts General Hospital Cancer Center in Boston, presented preliminary data from a phase I clinical trial in which the investigational therapeutic AG-270 was found to be safe and tolerable, and it showed clinical activity in patients with solid tumors that lacked both copies of the gene methylthioadenosine phosphorylase (MTAP).
The deletion of both copies of MTAP is seen in about 15 percent of all human cancers, according to Heist. Studies have shown that cancer cells with MTAP deletion are vulnerable to inhibition of the enzyme methionine adenosyltransferase 2A (MAT2A).
MAT2A plays a role in the production of the methyl donor S-adenosylmethionine (SAM). SAM is a substrate for the enzyme protein arginine methyltransferase 5 (PRMT5), which is found in all cells and plays a role in important cellular functions. Inhibiting the activity of PRMT5 by reducing the levels of its substrate, SAM, can slow tumor growth, Heist explained in an interview. Because healthy cells have not lost the MTAP gene, they would not be affected by reducing the levels of SAM.
AG-270 inhibits the activity of MAT2A. This reduces the levels of SAM, thereby inhibiting the activity of PRMT5 in cancer cells with MTAP gene deletion, slowing tumor growth.
Patients in this clinical trial had an advanced solid tumor with MTAP deletion. The doses of AG-270 tested in the trial ranged from 50 mg once a day to 400 mg once a day and 200 mg twice a day.
Treatment with AG-270 resulted in the reduction of plasma and tumor levels of SAM. One patient with high-grade neuroendocrine tumor of the lung had a partial response and eight other patients had stable disease. Even though the therapeutic produced some clinical responses, the investigators believe combining AG-270 with taxane-based chemotherapy could yield maximum benefit.
Adults with inoperable plexiform neurofibromas may benefit from a MEK 1/2 inhibitor
Most adults with neurofibromatosis type 1 (NF1), a genetic disorder, develop benign tumors called neurofibromas; about 20 to 50 percent develop benign plexiform neurofibromas, some of which can become aggressive cancers. Although these tumors are slow growing, they can grow very large and cause disfigurement, deformation, and enlargement of a body part, brain dysfunction, severe pain, and numbness.
NF1-associated plexiform neurofibromas are often inoperable, and the tumors often recur even when surgically removed, said Geraldine O’Sullivan Coyne, MD, PhD, staff clinician of the Developmental Therapeutics Clinic at the National Cancer Institute. Currently there are no U.S. Food and Drug Administration-approved therapeutics to treat this disease.
Preclinical studies have shown increased activity of the mitogen-activated protein kinase (MAPK) signaling pathway in NF1, and in a prior phase II clinical trial, inhibiting MEK, a player in the MAPK pathway, with a 25 mg twice daily dose of the investigational MEK inhibitor selumetinib yielded a response rate of 72 percent in children with inoperable plexiform neurofibromas. The responses had lasted for six months or more, in addition to yielding improvements in pain and motor impairment.
At the conference, O’Sullivan Coyne discussed data from a more recent phase II clinical trial, in which the efficacy of 50 mg twice daily selumetinib was tested in adult patients with NF1-associated inoperable plexiform neurofibromas. This dosage is lower than the standard dose of selumetinib given for adult patients with aggressive cancers, O’Sullivan Coyne noted in a media briefing. The study also recorded patient-reported outcomes. Preliminary data showed that 67 percent of the 21 evaluable patients had a partial response, with no disease progression observed as of this presentation. This was accompanied by significant improvement in patient-reported tumor pain intensity.
Data from several other clinical trials offer hope for cancer patients
More than 20 clinical trials presented at the conference tested a wide range of therapeutics against a variety of cancer types. These presentations, along with stimulating discussions on some of the most promising areas of cancer research, including new synthetic lethal molecular targets, new protein degradation technologies, new small molecule inhibitors, and new directions for immuno-oncology, made the conference a very exciting venue for all attendees, and offered hope to cancer patients across the globe.