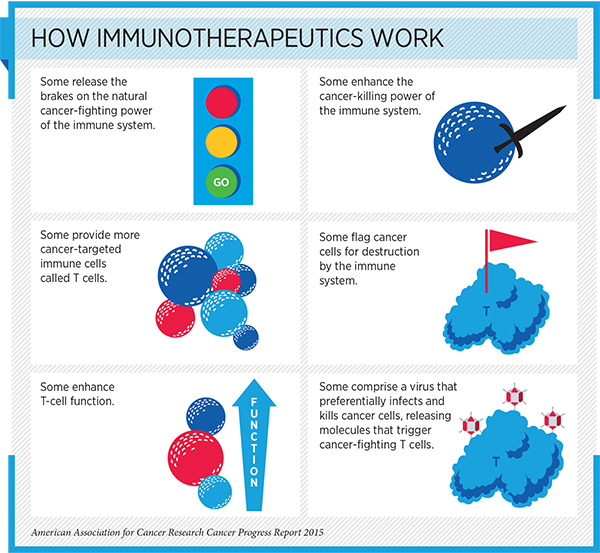
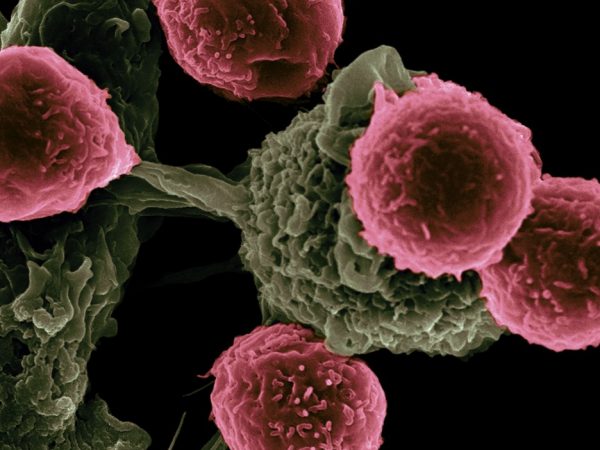
Immuno-oncology: AACR Partners with FDA to Accelerate the Pace of Progress
The current immunotherapy revolution that is changing the way we treat an increasing number of cancer types began in March 2011 when the U.S. Food and Drug Administration (FDA) approved the checkpoint inhibitor ipilimumab (Yervoy) for treating patients with unresectable or metastatic melanoma.
But the path to regulatory approval of ipilimumab was not easy. Although a lot was learned during the development of ipilimumab that helped ease the path to regulatory approval for other checkpoint inhibitors, many opportunities remain to enhance the process of developing the diverse array of immunotherapeutics, or immuno-oncology agents, currently being investigated. Some of these opportunities will be the focus of the FDA-AACR: Immuno-oncology Drug Development Workshop, to be held in Washington, D.C., Oct. 13–14.
Immunotherapy refers to treatments that harness the power of the immune system to fight cancer. Shown here are immune cells called T cells attacking a cancer cell.
Ipilimumab: Highlighting challenges in immuno-oncology drug development
Ipilimumab belongs to a class of immuno-oncology agents called checkpoint inhibitors. These drugs work by releasing brakes on immune cells called T cells, which have natural cancer-fighting ability.
In initial clinical trials, the effectiveness of ipilimumab as a treatment for melanoma was evaluated using the standard RECIST criteria for assessing investigational anticancer chemotherapeutics: ability to induce tumor regression within a specified time period, in this case 12 weeks. Based on this approach, ipilimumab did not look too promising, with only about 10 percent of patients having tumor responses. However, longer follow-up revealed that some patients had late responses and that ipilimumab significantly extended median overall survival.
The initial clinical trials with ipilimumab also revealed that the serious adverse events associated with ipilimumab treatment are very different from those seen with other anticancer agents. They are mostly inflammatory, such as skin rashes and colitis. Although they can be extremely serious, they can usually be managed.
Despite everything that was learned during the development of ipilimumab, research has taught us that not all immunotherapeutics work in the same way and that different agents can have distinct clinical efficacy and safety profiles. Therefore, continued and increased dialogue among researchers, regulators, and the pharmaceutical industry is essential if we are to provide the right patients access to the best immunotherapeutics that have been proven to be safe and highly effective in well-designed, well-conducted clinical trials at the earliest possible time.
FDA-AACR: Advancing Immuno-oncology Drug Development Workshop
This week, the American Association for Cancer Research (AACR) is partnering with the FDA to co-sponsor a two-day public workshop in Washington, D.C., that will bring together individuals representing many of the stakeholders in the immuno-oncology drug development community with the goal of enhancing the immuno-oncology drug development process.
“The recent successes of immunotherapy in a subset of cancers have demonstrated an urgent need for bettering the understanding of the mechanism of action of these medicines,” said workshop co-chair Jedd D. Wolchok, MD, PhD, the Lloyd J. Old/Virginia and Daniel K. Ludwig Chair in Clinical Investigation and chief of the Melanoma & Immunotherapeutics Service at Memorial Sloan Kettering Cancer Center (MSK) in New York. “This workshop seeks to both educate those who may be less familiar with the unique toxicities, dosing considerations, and clinical endpoints used and also stimulate dialogue between stakeholders in the academic, regulatory, and industry sectors who are focused on the development of these medicines.”
Suzanne L. Topalian, MD, professor of surgery and oncology at the Johns Hopkins University School of Medicine, and director of the Melanoma Program at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore, Maryland, who is one of the other workshop co-chairs, added, “The mechanism by which cancer immunotherapies work—directly targeting or engineering the patient’s immune system to enhance antitumor immunity—poses unique challenges for drug development. This workshop will address the entire drug development process through the lens of immuno-oncology: from preclinical evidence to dose-finding, toxicity monitoring and management, endpoint assessments, and clinical trial design.”
As highlighted by Wolchok and Topalian, the workshop program is designed to provide an interdisciplinary forum to foster robust scientific discussion on the need and potential for:
- new nonclinical toxicology models;
- modifications to traditional clinical safety monitoring;
- new efficacy endpoints and statistical analysis methods, and modifications of traditional endpoints; and,
- novel trial designs in immuno-oncology clinical trials.
“We hope that the convergence of key stakeholders at this workshop will crystallize ideas to accelerate immuno-oncology drug development in an already-changing biomedical and regulatory landscape,” emphasized Topalian, who is also an associate director of the Bloomberg~Kimmel Institute for Cancer Immunotherapy at Johns Hopkins.
Wolchok, who is also director of the Parker Institute for Cancer Immunotherapy center at MSK and associate director of the Ludwig Center for Cancer Immunotherapy at MSK, echoed these sentiments, saying, “It is our hope that participants will emerge with expanded knowledge of the field from all vantage points and that together we will find means to expedite clinical evaluation of the generation of immune-based treatments.”
For more information about the workshop and to register to attend in person or to join the live webcast, click here.