Breast Cancer
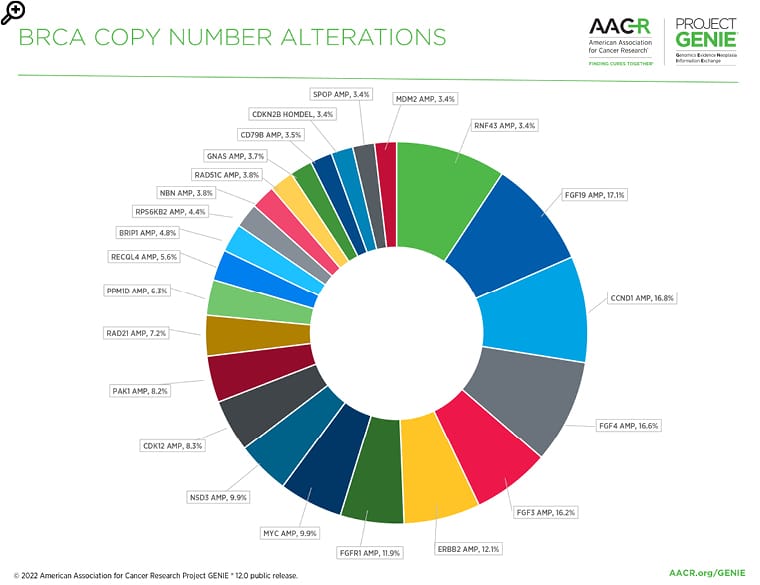
Figure 1: The most commonly mutated genes in the BRCA AACR Project GENIE registry in release 12.0-public.
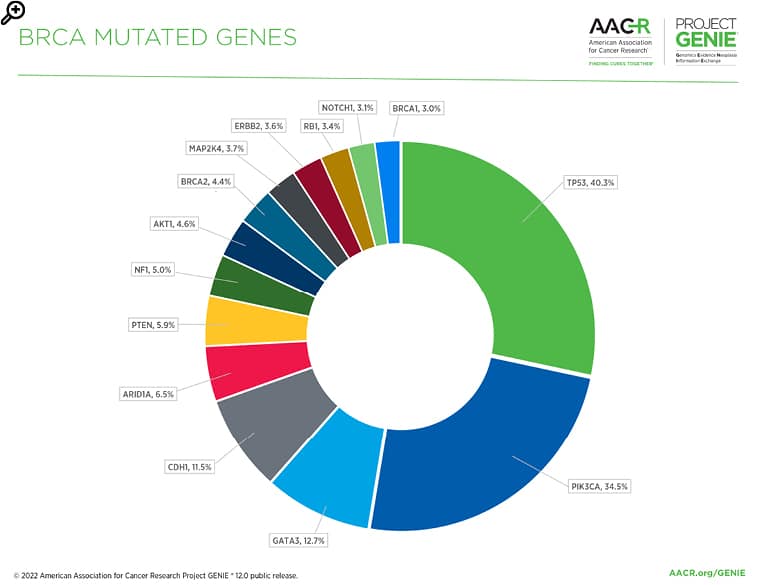
Figure 2: The most common gene fusions/structural variants in the BRCA AACR Project GENIE Registry in release 12.0-public.
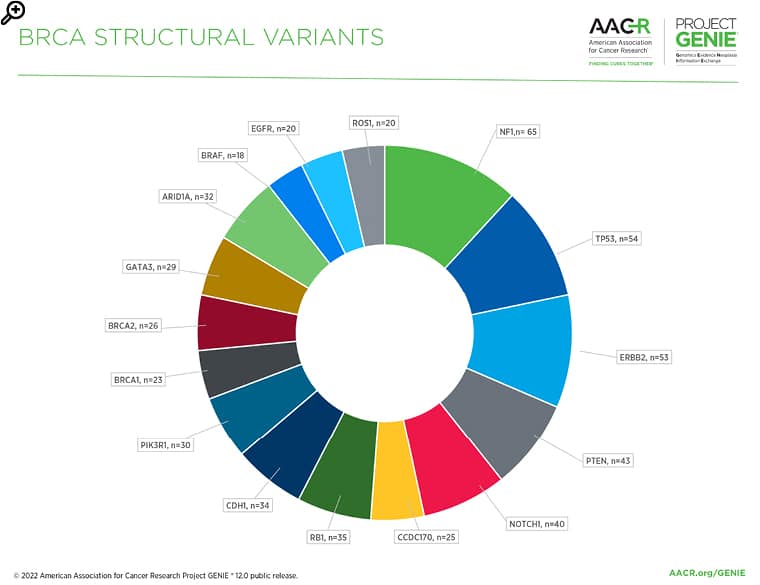
Figure 3: The most common copy number alterations in the BRCA AACR Project GENIE Registry in release 12.0-public.