Acute Myeloid Leukemia

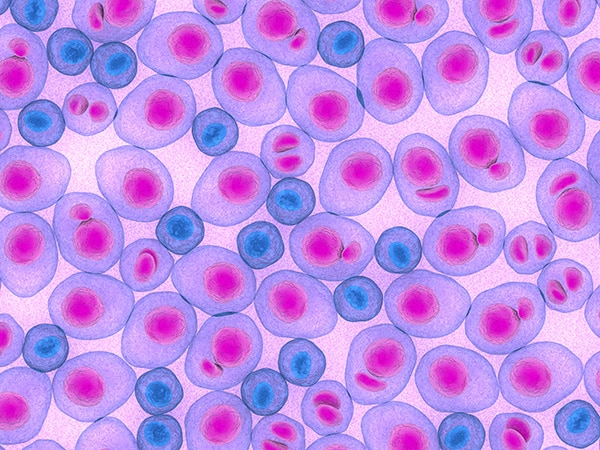
Acute myeloid leukemia (AML) is a cancer of the blood and bone marrow. It is the most common type of acute leukemia in adults. Normally, the bone marrow makes blood stem cells that become mature blood cells over time. A blood stem cell may become a myeloid stem cell or a lymphoid stem cell.
In AML, the myeloid stem cells usually become a type of immature white blood cell called myeloblasts (or myeloid blasts). The myeloblasts are abnormal and can build up in the blood and bone marrow so there is less room for healthy white blood cells, red blood cells, and platelets. These cells can spread outside the blood to other parts of the body.
Approximately 20,800 people in the United States will be diagnosed with AML and 11,220 will die from this disease in 2024, according to the estimates of the National Cancer Institute. Smoking, previous chemotherapy, and exposure to radiation may affect the risk of adult AML.
Adult Acute Myeloid Leukemia Treatment (PDQ®)Source: National Cancer Institute