How to Effectively Reach People with Your Science?
A Special Session at the AACR Annual Meeting 2024 offered researchers advice on better ways to communicate science to...

A Special Session at the AACR Annual Meeting 2024 offered researchers advice on better ways to communicate science to...

AACRoC in Brazil let young investigators learn from cancer research experts during programs in São Paulo and Ribeirão Preto.
March's picks include how synergy drives drug resistance in AML, new early detection tools, and more.

Three patients share their experiences about having Lynch syndrome.
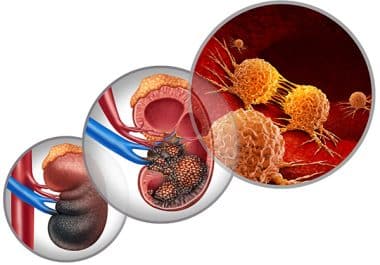
Researchers are exploring new options for kidney cancer treatments.

The AACR-Women in Cancer Research constituency group celebrates its 25th anniversary this year.
Researchers at the Blood Cancer Discovery Symposium presented data on multidrug resistance and clinical trial design.

Cody Wolf was one of 19 young scientists who visited Washington for AACR's Early-career Hill Day.

Four of the 2023 GSITA recipients explain how the program has benefited their careers.

Event cochair Dr. Michael Caligiuri shares how attendees can form connections to take science from bench to market.
Lobular breast cancer accounts for 15% of all breast cancer cases, but it is understudied.