Experts Forecast Cancer Research and Treatment Advances in 2020
Around 400 BC, the Greek physician Hippocrates is believed to have first described tumors as “cancer,” using the words “karkinos” or “karkinoma,” which stem from the Greek word for “crab.” Today, the word “cancer” is used to describe hundreds of different types of this disease. As our understanding of cancer continues to grow, prevention, detection, and treatment advancements continue to evolve.
While enormous progress has been made in the field of oncology this past decade, the most striking development has been the incredible growth of the field of immunotherapy. As highlighted in this recent post, the U.S. Food and Drug Administration (FDA) has approved immunotherapeutic regimens for two dozen cancer types so far, and thousands of clinical trials are currently evaluating immunotherapeutic approaches for the treatment of cancer. The field of precision medicine has also witnessed extraordinary advancements: Just this past year, the FDA approved 11 new anticancer therapeutics, and all of these are molecularly targeted agents.
The breakthroughs in cancer research and treatment, however, do not benefit everyone equally. To educate both the public and Congress about cancer health disparities and to advocate for increased federal funding for this important area of research, the AACR is publishing its first ever report on cancer health disparities in 2020.
As a new decade begins to unfold, what advancements can we expect in the field of oncology? We talked with immunotherapy expert and AACR Women in Cancer Research (WICR) member Padmanee Sharma, MD, PhD; AACR board member, Fellow of the AACR Academy, and precision medicine expert Martine Piccart, MD, PhD; AACR treasurer, AACR Past President, Fellow of the AACR Academy, and cancer prevention and interception expert William Hait, MD, PhD; and AACR board member and cancer disparities expert Marcia Cruz-Correa, MD, PhD; to discuss their unique perspective and to predict cancer research progress in 2020.
Immunotherapy advances in 2020
Finding ways to unlock the immune system to better recognize and attack cancer cells has been a major focus in the oncology field in the past decade. Specifically, drugs that block the immune checkpoints CTLA-4 or PD-1/PD-L1, known collectively as immune checkpoint inhibitors, have been approved by the FDA to treat over a dozen different cancer types. “With the use of immune checkpoint inhibitors, we’ve been able to see that the immune system can treat many different types of cancer, regardless of where they originate,” began Padmanee Sharma, MD, PhD, professor in the Department of Genitourinary Medical Oncology and Immunology at The University of Texas MD Anderson Cancer Center. “However, not all patients respond to this therapeutic strategy, and not every tumor type responds in the way that we expect,” she said. “We must work diligently to improve the number of patients that can benefit from immunotherapeutic approaches.”

One key area of research will focus on understanding why some tumor types – such as pancreatic cancer or glioblastoma – are more resistant to immune checkpoint inhibitors, predicts Sharma. While the field has centered on targeting T-cell pathways, which drive the antitumor response, many tumor types that do not respond to immune checkpoint inhibitors are infiltrated by myeloid cells rather than T cells, she explained. “Myeloid cells have alternative pathways that suppress the immune response, so targeting these cells and other immunosuppressive pathways may be the next step to improve immune checkpoint inhibitor efficacy,” Sharma noted.
Another research avenue that could increase the number of patients responding to immunotherapy is the investigation of combinatorial treatments. “Immune checkpoint inhibitors can be combined with a host of other therapeutic strategies,” Sharma said. She highlighted the use of drugs that target tumor cells directly, such as chemotherapies, radiation therapies, or targeted therapies, as a “primer” for immune checkpoint blockade. “When tumor cells die, they are taken up by antigen-presenting cells, which present the offending antigen to the T cells as part of the immune response,” she explained. “An immune checkpoint inhibitor can then enhance the T-cell killing of the remaining cancerous cells.”
Combinatorial immunotherapies, such as targeting both the CTLA-4 axis and the PD-1/PD-L1 axis simultaneously, are also being explored. “One might think that individual immune checkpoint inhibitors are interchangeable, but they’re not,” stressed Sharma. “Basic science has taught us that the CTLA-4 pathway works much earlier during an immune response compared with the PD-1/PD-L1 pathway. The combined inhibition of these immune checkpoints has been approved for multiple tumor types, such as melanoma and renal cell carcinoma, and we’ll continue to see this approach used in other tumor types as well.”
However, combinatorial approaches may be accompanied by increased toxicity. “The challenge is, once we unleash the immune response, we’re releasing it throughout the entire body,” Sharma noted. “As the immune system attacks the tumor cells and the tumor antigens, it may also attack self-antigens, which could be present in any part of the body, resulting in different inflammatory conditions.” As such, a major area of research will focus on understanding and mitigating immune-related adverse events associated with checkpoint inhibitors, Sharma predicts. “We are trying to understand the underlying mechanisms of these adverse events and to determine the specific self-antigens that are being targeted by the immune system,” she said. “We would then need to develop strategies to better manage these toxicities or develop drugs that minimize responses against self-antigens without comprising the antitumor effects of the checkpoint inhibitor.”
Sharma believes that using gene editing techniques like CRISPR to further modify chimeric antigen receptor (CAR) T cells to better attack cancer cells or to enhance T-cell survival is an exciting area of research that has come to the fore. “Researchers are currently investigating the use of CRISPR to disrupt PD-1 in order to help CAR T cells become more effective,” Sharma said. This strategy, conceptually, is like combining checkpoint inhibition with CAR T-cell therapy, she noted. “In addition to altering immunosuppressive pathways, other modifications of CAR T cells to enhance their survival could be considered, as some CAR T cells do not survive long enough in the body to generate an effective and durable response.”
Challenges in the field include the identification of biomarkers for selecting patients for immunotherapy, Sharma said. “Finding biomarkers for immune checkpoint inhibitors is difficult because the immune system is very dynamic,” she noted. “The fields of genomic medicine and immunotherapy will need to come together to help identify those who are most likely to respond to immune checkpoint inhibitors.” We’re seeing progress in this area, such as the tissue-agnostic approval of pembrolizumab for certain patients with microsatellite instability (MSI)-high or mismatch repair deficient (dMMR) solid tumors, Sharma said. “I think that we’ll need to use biomarker combinations – one biomarker that relies on specific mutations found in the tumor, and another biomarker that relies on the activation of the immune response – to better identify patients that will respond to immunotherapeutic approaches,” she concluded.
Precision medicine advances in 2020
“Precision medicine – the personalized tailoring of treatment strategies based on an individual’s cancer – is something that we need to strive for, but we’re not there yet,” began Martine Piccart, MD, PhD, cofounder of Breast International Group and Scientific Director at the Institut Jules Bordet in Brussels.

As the field stands now, clinicians are able to use “stratified medicine” for some cancer types, Piccart explained. By using a handful of biomarkers, Piccart is able to stratify breast cancer patients as having a low or high likelihood of responding to a particular therapeutic regimen.
However, advances in the field are driving progress towards the ultimate goal of precision medicine, noted Piccart. She began by talking about the incredible responses witnessed with the advent of immunotherapy, but noted that responses are not seen among the majority of patients.
“An area of intense focus will center on why certain patients are not benefiting from immunotherapy treatments,” Piccart said. “I see a great potential in the combined examination of the genomic landscape of these cancers along with the tumor microenvironment. I think this strategy will lead to enhanced tools to help predict not only who is going to respond to a particular treatment, but also which treatment you should prioritize for your patient.”
Looking at mutations or gene amplifications present in the cancer cells with technologies such as DNA/RNA sequencing is an important step forward for patients, said Piccart. “I cannot imagine that something great is not going to be generated by this type of research,” she noted. Further, the monitoring of patients using liquid biopsy approaches will help to generate a more complete picture of an individual’s cancer and will allow for the monitoring of disease evolution in “real time,” Piccart said. “With the help of bioinformaticians, we can develop really efficient tools for precision medicine, which will help us to choose the right treatment for the right patient at the right time. This is one area of the field where I’m very excited,” said Piccart.
Piccart noted that advances in imaging technologies are also likely to foster rapid progress in the field. “What fascinates me is that there are a lot of novel biological tracers that can be utilized to accurately visualize cancerous cells in the body,” she noted. The technology of linking antibodies to a radioisotope, as is used in positron emission tomography (PET), can help radiologists distinguish whether tumors are expressing a particular antigen in real time, thereby guiding efficient treatment decisions. As an example, the utilization of HER2 PET using zirconium-labelled trastuzumab allows clinicians to discern whether or not metastatic breast cancer lesions are able to bind to the drug, which can help determine if this approach is a viable option for advanced disease, Piccart explained. “This type of strategy is going to explode in the coming years,” she predicts.
Another key area of growth will be enhanced use of big data approaches, Piccart said. “It’s absolutely clear that using big data could have a dramatic impact on the way that we are going to treat future patients,” she noted. “If we can find a way to best compile these data – data about the genetic makeup of specific cancers, the comorbidities of patients, and how these patients respond to certain treatments – this will change the way we treat cancer. It’s an extremely important field with huge potential.” Key hurdles, such as the best ways to share these data, along with regulations governing the use of such data, will need to be overcome to fully utilize this growing asset, Piccart noted.
The use of artificial intelligence (AI) will continue to expand, predicts Piccart, especially in the analysis of microscopy images. “Pathology slides contain a vast amount of information, and some features of the cancer tissue can’t be picked up by the human eye,” Piccart said. “Compiling these images and linking image features with patient outcome could help us predict who will benefit from certain treatments or direct patients who would be better served by a new drug in a clinical trial than the standard of care.”
While collaborations play an important role in the rapid progress of cancer research and technological advancements, challenges lie within the culture of collaboration, noted Piccart. “The current system relies on publications for individuals’ progress with their career and does not put enough emphasis on collaborative work, making it challenging for researchers to share data and collaborate.” We need some changes to the system, including facilitating more funding for collaborative research, she emphasized.
“Nonetheless, the advent of new technologies has allowed us to develop specialized, less aggressive treatments for many patients, and I can see a future where true precision medicine will be possible,” concluded Piccart.
Cancer prevention and interception advances in 2020
“In the coming years, I anticipate that there will be a greater appreciation that the largest payoff for the health of humanity will come from cancer prevention,” said William Hait, MD, PhD, who is the global head of Johnson & Johnson External Innovation. “Currently, the majority of the research community is invested in understanding established cancers, with less emphasis on preventing cancers from becoming established,” he continued.

“I would anticipate that as people incorporate this realization into their thinking, there will be a global movement to encourage funding agencies, advocacy groups, and public health groups to ensure that a greater percentage of the research dollar goes toward three core components: cancer prevention, cancer interception, and early detection to increase cures,” Hait said. “If we can prevent cancers, intercept a cancer-causing process, or detect cancers at an earlier stage, we will realize better outcomes.”
A major area of focus in the field should be the validation of actionable targets in premalignancies such as the work of the Precancer Atlas, said Hait, which aims to characterize premalignant lesions and their evolution into cancer. “We have massive amounts of biological information about first diagnoses, first relapses, second relapses, and so on, but we have far less information about targets and pathways present in premalignant conditions,” he said.
A second research area that requires more attention is the need for validated surrogate endpoints for clinical trials of premalignancy, Hait continued. Endpoints such as cancer incidence or survival require extended years of expensive follow-up, and currently there are few validated surrogate endpoints that can indicate that a specific prevention or intervention strategy will prevent incidence or extend survival, he said. Hait cited the lowering of low-density lipoprotein (LDL) cholesterol as an example of both a validated pharmacodynamic biomarker and surrogate endpoint for cardiovascular disease. “We need similar biomarkers to provide early readouts to see if prevention/interception drugs are working in oncology.”
There is also a great need for accurate diagnostic tests to identify individuals that are harboring premalignancies, noted Hait. Further, if we could identify those with a premalignant condition, a test to identify individuals at the highest risk of progression is also an unmet need, he said. “We’re making progress in this area for patients with smoldering myeloma – we are able to stratify those with this premalignancy as having low or high risk for developing multiple myeloma, which is a huge advancement in the field,” Hait explained. But there are very few examples where those types of stratifications exist, he added. “Such tests will allow clinicians to focus on interventions for those at the highest risk, and spare low-risk individuals from unnecessary interventions,” he added.
Hait said behavioral science will receive increased interest in cancer prevention. As an example, he highlighted the fact that most people understand that cigarette smoking is a major cause for lung cancer, yet many people still choose to smoke. “What is happening in someone’s brain to override the evidence that shows us that smoking is bad for you?” posed Hait. “We can come up with several descriptive causes of smoking, such as stress or a desire to lose weight. But we don’t understand the underlying biological mechanisms that lead people to begin smoking in the first place. There’s a need for far greater research in this particular area of cancer prevention.”
Hait pointed to certain areas of progress in cancer prevention so far, such as a decline in the rates of cigarette smoking; invention of the human papillomavirus (HPV) vaccine, which can help prevent cervical and head and neck cancers, among others; and the availability of curative treatments for hepatitis C, which can help reduce the incidence of liver cancer.
However, “we still have a lot of work to do,” Hait concluded. “A greater focus on approaches to prevention and interception is needed if we hope to generate the knowledge that will ultimately lead to significant progress in reducing the number of people diagnosed with cancer and increasing the number who are cured.”
Disparities research advances in 2020
“Recently, we have seen numerous studies that highlight the pervasiveness of disparities in cancer incidence, prevalence, and survivorship across diverse populations,” said Marcia Cruz-Correa, MD, PhD, professor of medicine and biochemistry at the University of Puerto Rico Cancer Center. “However, these studies have yet to really impact large changes in health policy.”

Most research on cancer health disparities has focused on descriptive epidemiology and has not delved into the underlying reasons for the observed disparities, Cruz-Correa noted. “Many studies have evaluated factors that are related to limited access to health care, along with other social determinants,” she said. “However, the mechanisms underlying disparities in molecular biology, which may or may not be driven by socioeconomic factors, need to be explored and systematically studied.”
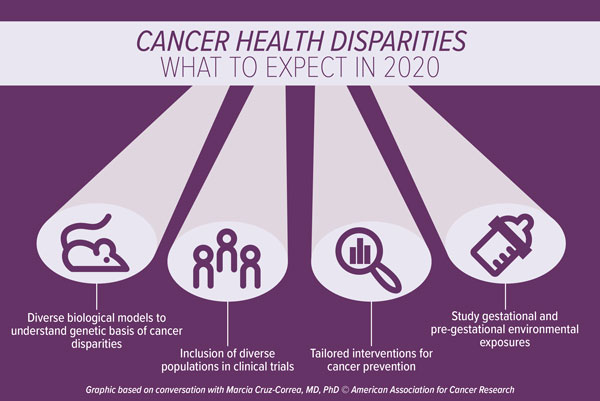
Cruz-Correa predicts that there will be an increased focus in the generation of diverse animal models and cell lines to help study disparities observed in cancer incidence and in response to therapies. “Genomic diversity in biological models will drive research to examine molecular aspects of cancer-related disparities,” she noted.
“The representation of minority populations in oncology clinical trials is extremely limited and unbalanced,” Cruz-Correa said. Indeed, pooled data from 2003 to 2016 indicate that non-Hispanic white patients comprise more than 80 percent of oncology clinical trial participants and the enrollment of African American and Hispanic patients in such trials has declined compared with historical data from 1996 to 2002. Clinical trials need to expand to include diverse populations, including different races, ethnicities, ages, genders, and socioeconomic statuses, she noted. “The inclusion of different populations in clinical trials will help expand our understanding of the biological profile of tumor pharmacogenetics, as well as the unique factors associated with cancer outcomes among diverse populations,” Cruz-Correa said.
In addition, environmental exposures, including gestational and pre-gestational exposures, should also be studied, as they may help to elucidate risk factors and potential preventive actions, noted Cruz-Correa. “Several studies have examined how certain genes are epigenetically modified by methylation that occurs during conception or during the intrauterine phase as a result of dietary exposures or lack of certain nutrients,” Cruz-Correa said. “As such, new research should analyze how the dietary habits of expecting mothers may modify the expression of genes and impact the function of important tumor suppressor genes.”
Cruz-Correa believes that intervention studies focused on cancer prevention tailored to diverse and minority populations will also be an important area of research. “Prevention of cancer is the holy grail that we all strive for, and there are known factors that increase the risk of cancer,” she said. “Interventions to reduce or eliminate those risk factors – such as HPV vaccination, tobacco control, and cancer screening – can help prevent cancer.” However, these advances are not equally adopted by all segments of the population, Cruz-Correa stressed. “Differences in adherence to prevention measures result in differences in cancer outcomes, thus reducing health equity. As a community of cancer researchers, we need to work toward developing culturally sensitive methods for the diverse population that we serve. Further, we need to work with health policy experts to eliminate barriers related to insurance coverage and access for the most vulnerable.”
Another important area is the availability of cancer genetic testing for individuals with cancer and their family members, said Cruz-Correa. “Close to 10 percent of all cancers are hereditary and may be preventable with germline genetic testing and identification of mutation carriers. The availability of genetic counseling/testing is key to preventing hereditary cancer and improving equity in cancer care.”
Increasing and retaining minority investigators has been a challenge in the field, noted Cruz-Correa. “It is key to continue to promote national programs that provide opportunities for career development, mentoring, and sponsoring of minority investigators,” she said. “Increasing the number of investigators from underserved groups will increase the pool of scientists focusing on cancer disparities in minority populations.
“There continues to be a limited awareness of cancer health disparities, which is a challenge,” noted Cruz-Correa. “While most studies have focused on differences across the continuum of cancer care, the translation of the observed differences into health policies and/or intervention programs aimed at decreasing disparities is limited and lags behind significantly,” she said. “Data on cancer disparities need to be presented to all stakeholders including health insurance companies, government officials, and the lay public to create awareness, and stir debates. This could help ignite the community to develop interventions to decrease disparities and promote equity in cancer care. It will take a village to move the pendulum and decrease cancer health disparities for all.”