Editors’ Picks, April 2024: Targeting Metabolism, KRAS, and More
April picks include metabolic interventions for acute myeloid leukemia, new inhibitors of KRAS signaling, and more.

April picks include metabolic interventions for acute myeloid leukemia, new inhibitors of KRAS signaling, and more.
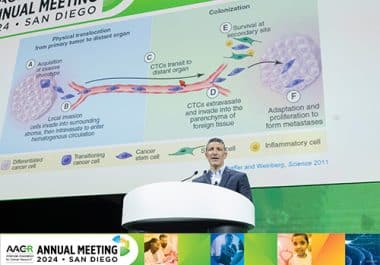
A plenary session at the AACR Annual Meeting 2024 explored how metastases adapt to—and help shape—their new environments.
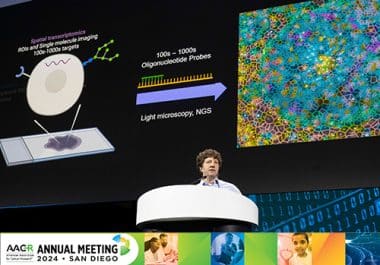
At the AACR Annual Meeting 2024, researchers discussed innovative approaches to decipher the complexities of tumor ecosystems.

A session at the AACR Annual Meeting 2024 offered advice on better ways to communicate science to the public....

The AACR Annual Meeting 2024 Opening Plenary showed how researchers are inspiring science, fueling progress, and revolutionizing care.

Researchers at the AACR Annual Meeting 2024 presented the latest findings regarding two personalized neoantigen cancer vaccines.

A poster at the AACR Annual Meeting 2024 examined a video that increased awareness of prostate cancer.

Experts discussed "Discovery Science in Early Cancer Biology and Interception" at a plenary during the AACR Annual Meeting 2024.

The 2024 AACR June L. Biedler Prize for Cancer Journalism winners were honored during the AACR Annual Meeting 2024.
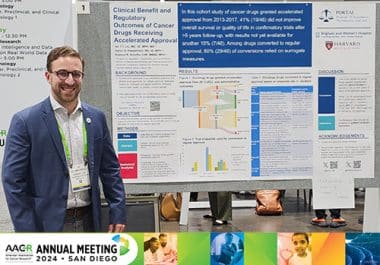
Dr. Edward Cliff presented a poster on the clinical benefit of cancer drugs granted accelerated approval.

The AACR Annual Meeting 2024 Opening Ceremony showcased impactful initiatives impacting cancer research.