Non-Small Cell Lung Cancer
Figure 1: The most commonly mutated genes in non-small cell lung cancer (NSCLC) in the AACR Project GENIE registry in release 12.0-public. Inset displays the distribution of KRAS G12 variants in non-small cell lung cancer in the AACR Project GENIE registry.
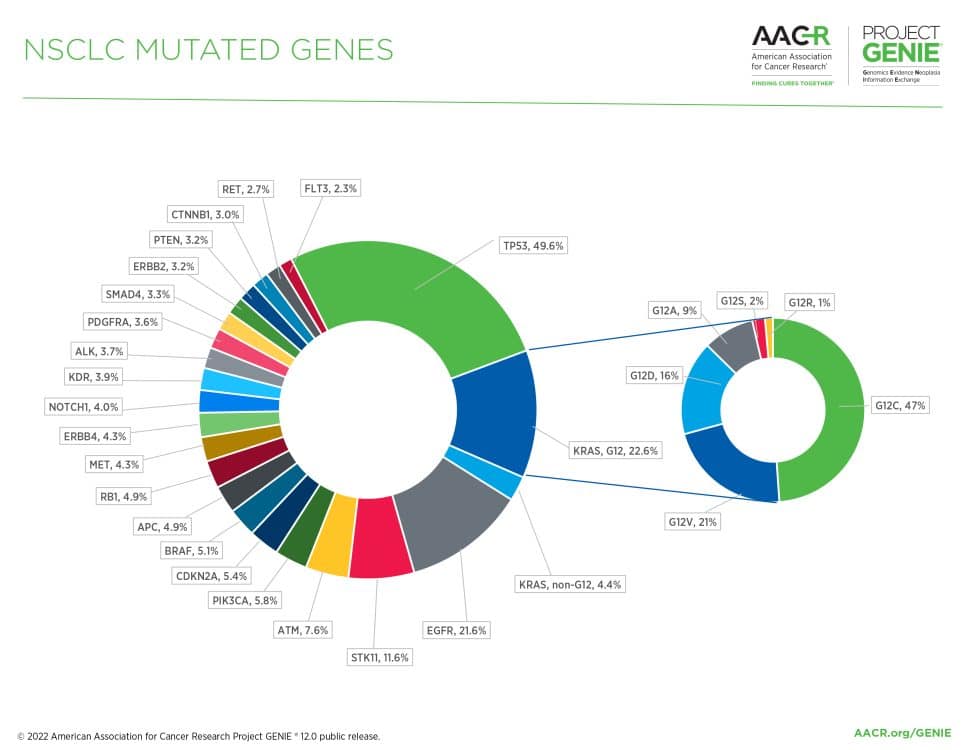
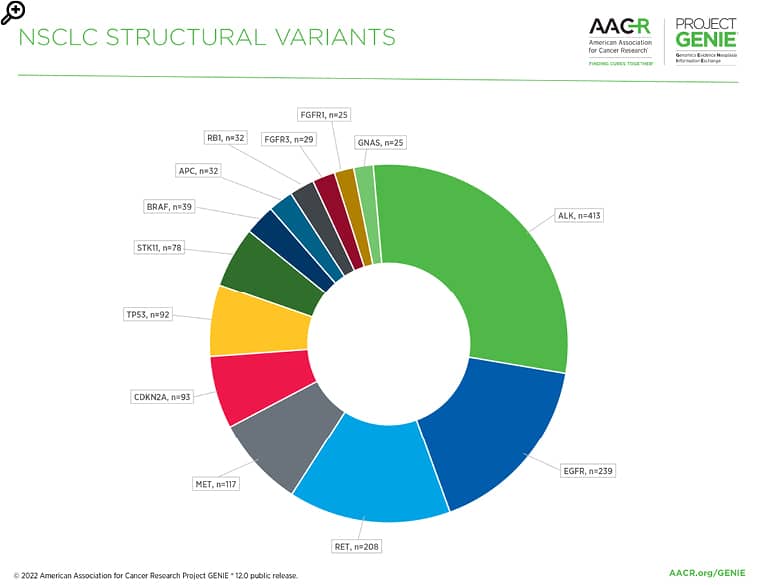
Figure 2: The most common gene fusions/structural variants in non-small cell lung cancer in the AACR Project GENIE Registry in release 12.0-public.
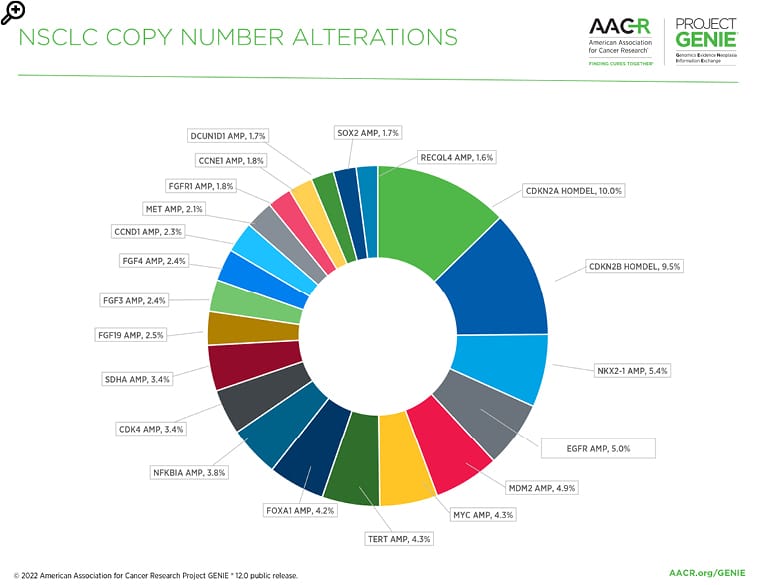
Figure 3: The most common copy number alterations in non-small cell lung cancer in the AACR Project GENIE Registry in release 12.0-public.