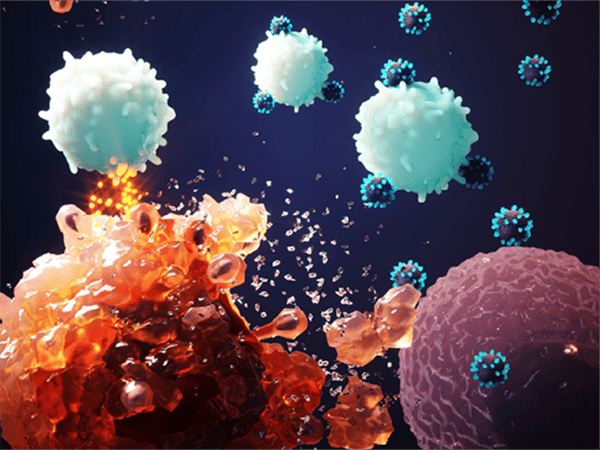
Cancer Immunotherapy: Breaking Through to the Standard of Care
It seems that hardly a week goes by without there being a report of new breakthroughs in cancer immunotherapy.
At the start of the year, the focus was on the decision of the editors of Science to choose cancer immunotherapy as Breakthrough of the Year for 2013. In February, researchers at Memorial Sloan Kettering Cancer Center in New York published a study showing that 14 of 16 adults with relapsed or refractory acute lymphoblastic leukemia (ALL) had a complete response after treatment with an investigational cancer immunotherapy. And so it has gone on. Most recently, the U.S. Food and Drug Administration (FDA) granted breakthrough therapy designation to another investigational cancer immunotherapy, CTL019, for the treatment of pediatric and adult patients with relapsed/refractory ALL.
The excitement surrounding cancer immunotherapy was evident June 18, when a Twitter chat titled “The Promise of Immunotherapy,” organized by the American Association for Cancer Research (AACR) in partnership with Time magazine, the Mayo Clinic Cancer Center, and the Cancer Research Institute, reached an estimated 1.3 million individuals.
But what is cancer immunotherapy, how does it work, and why are people so excited about it?
Cancer immunotherapy, which was featured as a key advance in patient care in the AACR Cancer Progress Report 2013, refers to treatments that can unleash the power of a patient’s immune system to fight his or her cancer. Decades of research have provided us with immense scientific insight into the immune system and how it interacts with cancer cells. This is what is allowing us to design increasing numbers of anticancer therapies that harness the immune system in different ways. It is also what is underpinning the groundswell of clinical trials testing cancer immunotherapies that we have seen in the past few years.
One of the reasons cancer immunotherapies have generated such excitement is that some have yielded dramatic and long-lasting responses in a number of patients. For example, Andrew Messinger (a cancer survivor featured in the AACR Cancer Progress Report 2013), who has metastatic melanoma – a disease that has an overall five-year survival rate of just 16 percent – is continuing to benefit from the cancer immunotherapy ipilimumab (Yervoy) five years after his first dose.
Ipilimumab is an example of a type of cancer immunotherapy known as a checkpoint inhibitor. These cancer immunotherapies work by releasing the ‘brakes’ on immune cells called T cells, which are naturally capable of destroying cancer cells. The brake released by ipilimumab is called CTLA4.
As highlighted in the AACR Cancer Progress Report 2014, which will be released in mid-September, a number of investigational cancer immunotherapies target a second T-cell brake called PD-1. One of these, pembrolizumab, is under review at the FDA as a potential treatment for metastatic melanoma. Another, nivolumab, received regulatory approval as a treatment for unresectable melanoma in Japan in July. Promising early results among patients with a number of other types of cancer, including non-small cell lung cancer and non-Hodgkin lymphoma, have been reported for PD-1-targeted cancer immunotherapies, but the data need to be confirmed in larger cohorts.
Despite all the excitement surrounding cancer immunotherapies, ipilimumab is one of the few currently approved by the FDA – it was approved for the treatment of metastatic melanoma in March 2011. As a result, most cancer immunotherapies remain investigational treatments, meaning that they have not yet been approved by the FDA and are still in development.
Fortunately, researchers are continuing to uncover new information about how the immune system functions. Thus, we can expect to see novel immunotherapies and new ways to use those that we already have in the future. Some of the most promising research in this area will be discussed at the AACR special conference Tumor Immunology and Immunotherapy: A New Chapter, which is being held in Orlando, Florida, Dec. 1-4. This conference is being put together in part by the AACR’s Cancer Immunology Working Group, which helps provide a forum for immunologists and non-immunologists to meet, exchange knowledge and ideas, and discuss the present status and future promise of cancer immunology.