Biomarkers and Liquid Biopsy for Early Detection of Colorectal Cancer
Colorectal cancer is one of the few cancers for which we have screening tests that have been shown to reduce disease incidence and mortality. However, it remains the fourth most commonly diagnosed cancer in the United States, and researchers are actively trying to develop ways to improve early detection of colorectal cancer. As discussed previously on this blog, Stand Up To Cancer (SU2C) and the Dutch Cancer Society brought together a group of researchers to work on this issue. AACR is the proud Scientific Partner for SU2C, and for colorectal cancer awareness month, we asked the co-leader of the SU2C – Dutch Cancer Society Dream Team on Molecular Early Detection of Colorectal Cancer, Victor Velculescu, MD, PhD, about the work of the Dream Team and other efforts in this field of cancer research.
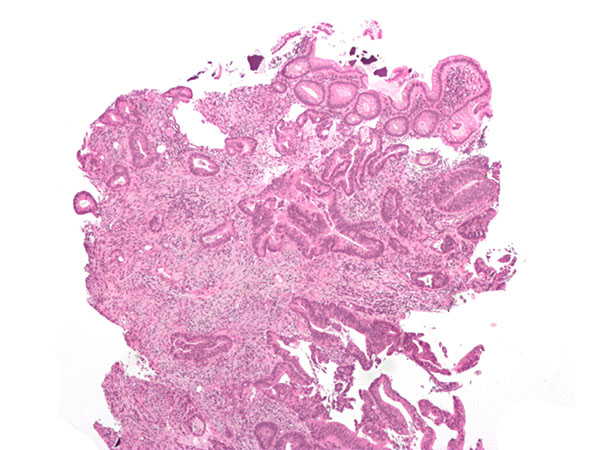
Invasive adenocarcinoma (the most common type of colorectal cancer). The cancerous cells are seen in the center and at the bottom right of the image (blue). Near normal colon-lining cells are seen at the top right of the image.
How big of a problem is colorectal cancer (CRC)?
Among American men and women combined, it is the second-leading cause of cancer death, after only lung cancer. One American in 22 will be diagnosed with colorectal cancer at some point in his or her lifetime. We will have more than 49,000 deaths from colorectal cancer this year.
What’s being done to improve early detection of colorectal cancer?
We’ve done a great deal of laboratory research on DNA-based biomarkers. We know that a tumor or precancerous lesion will often shed DNA into the bloodstream that has telltale mutations. Because it is in the bloodstream, this is called circulating tumor DNA. We’re studying so-called liquid biopsies, which are blood samples from patients, and running some very sophisticated genetic analyses to look for the mutations. Scientists all over the world are working on this type of research, including our Stand Up To Cancer Dream Team, to help develop new ways to screen populations for cancer and detect recurrent cancers in patients earlier.
Scientists all over the world are working on this type of research, including our Stand Up To Cancer Dream Team, to help develop new ways to screen populations for cancer and detect recurrent cancers in patients earlier.
How does the liquid biopsy help the patient?
We’re still working on the liquid biopsy approach [that is, the analysis of circulating tumor DNA isolated from blood samples from patients] and the best way to use this technology for patients. If a liquid biopsy helps doctors to identify a tumor at an early stage, then surgery can remove the tumor and hopefully provide a cure. The liquid biopsy could also help identify patients who might benefit from extra treatments, like chemotherapy, after surgery. In early-stage colorectal cancer, 80 percent of patients will be fine without chemotherapy after surgery. But there’s 20 percent who need it. The liquid biopsy could potentially help identify the 20 percent so they can get the extra treatments they need.
In the future, liquid biopsies could also help doctors select targeted therapies based on the genetic mutations found in individual patients.
What else is being done for early detection of colorectal cancer?
I am co-leader of a Dream Team on early detection of colorectal cancer sponsored by Stand Up To Cancer and the Dutch Cancer Society. The leader of the team, Gerrit A. Meijer, MD, is based in the Netherlands. His team is working on an improved stool test that is also based on highly sensitive molecular testing for cancer biomarkers. The idea is to expand the number and variety of biomarkers that are available and to pick the best ones to use in population screening for colorectal cancer. We hope to make that kind of very sensitive testing available to patients in everyday life. There’s a large national population-screening program for colorectal cancer in the Netherlands already, so the new tests will be compared with the existing test and hopefully validated.
We hope to make that kind of very sensitive testing available to patients in everyday life.
Is colonoscopy still the gold standard?
Colonoscopy is a very powerful method of screening, but it is expensive, it has to be done in the doctor’s office, and it requires preparation that some people find difficult. So if we can develop improved stool sample tests or blood tests, then we will have more ways to attack the problem of screening a large population for colorectal cancer or precancerous lesions.
Finally, what’s the future of colorectal cancer detection and care?
Like most other forms of cancer, colorectal cancer is largely a disease of the aging. Ninety percent of new cases occur in people 50 years of age or over. Of course, that age group is growing as the “baby boom” generation ages, so we will be seeing many more cases. We’re also seeing an increase in colorectal cancer in younger patients, even people in their twenties and thirties. That’s why we need to improve screening and make it more available to the public.
Victor Velculescu, MD, PhD, is a member of the American Association for Cancer Research (AACR) board of directors, co-leader of the SU2C – Dutch Cancer Society Dream Team on Molecular Early Detection of Colorectal Cancer, and co-director Cancer Biology and professor of Oncology and Pathology at the Johns Hopkins Kimmel Cancer Center. His research interests are focused on genomic analyses of human cancer and using such information to understand the underlying biology of cancer and to identify new diagnostic and therapeutic approaches. He is the recipient of several awards for his work including the AACR Award for Outstanding Achievement in Cancer Research (2009) and the AACR Team Science Awards for Pancreatic (2013) and Brain Cancer Research (2014).
This post was updated on March 24, 2016, at 11:35am.




